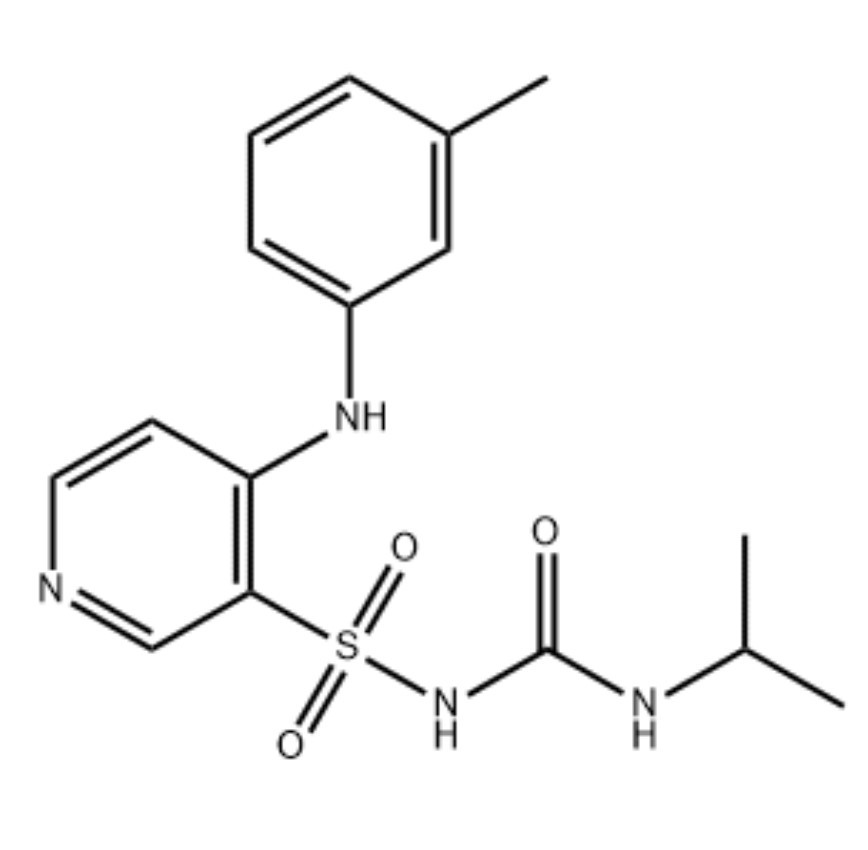
Description
Usage:
Torasemide is a novel loop diuretic launched in 1993 after a 12-year gap from the last diuretic introduction. It is indicated for the treatment of hypertension and edema associated with chronic congestive heart failure, renal disease and hepatic cirrhosis. Torasemide exerts its major diuretic activity on the thick ascending limb of the Henle's loop to promote rapid and marked excretion of water, Na+, Cl-, and to a lesser extent,K+ and Ca2+. Compared with other loop diuretics such as furosemide, torasemide has a stronger antihypertensive action, a higher bioavailability, a longer duration of action that is independent of the renal function, and has no side effects such as paradoxical antidiuresis.
| Product name | Torsemide | ||
| Reference | USP | ||
| TEST | SPECIFICATION | RESULT | |
| Appearance | White or off-white crystalline powder | Off-white crystalline powder | |
| Identification | The IR spectrum of the substance corresponds to that of the reference standard. | Conforms | |
| The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay | Conforms | ||
| Water | ≤1.0% | 0.1% | |
| Residue on ignition | ≤0.1% | <0.1% | |
| Assay (HPLC) | 98.0~102.0%(calculated on anhydrous basis) | 99.9% | |
| Related substances(HPLC) | Impurity A≤0.5% | <RL(RL=0.05%) | |
| Impurity C≤0.2% | <RL(RL=0.05%) | ||
| Impurity B≤0.3% | <LOD(LOD=0.005%) | ||
| Unspecified impurities≤0.10% | <LOD(LOD=0.009%) | ||
| The total of unspecified impurities≤0.20% | <LOD | ||
| Total impurities≤1.0% | <RL | ||
| Residual solvents(GC) | Ethanol≤5000ppm | <LOD(LOD=30ppm) | |
| Methanol≤3000ppm | 266ppm | ||
| Dichloromethane≤600ppm | <LOD(LOD=60ppm) | ||
| N,N-dimethylformamide≤880ppm | <LOD(LOD=29ppm) | ||
| Conclusion | Complies with USP | ||